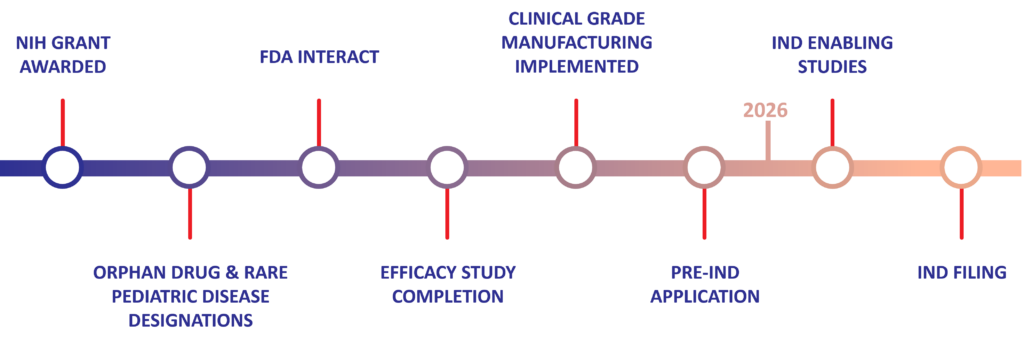
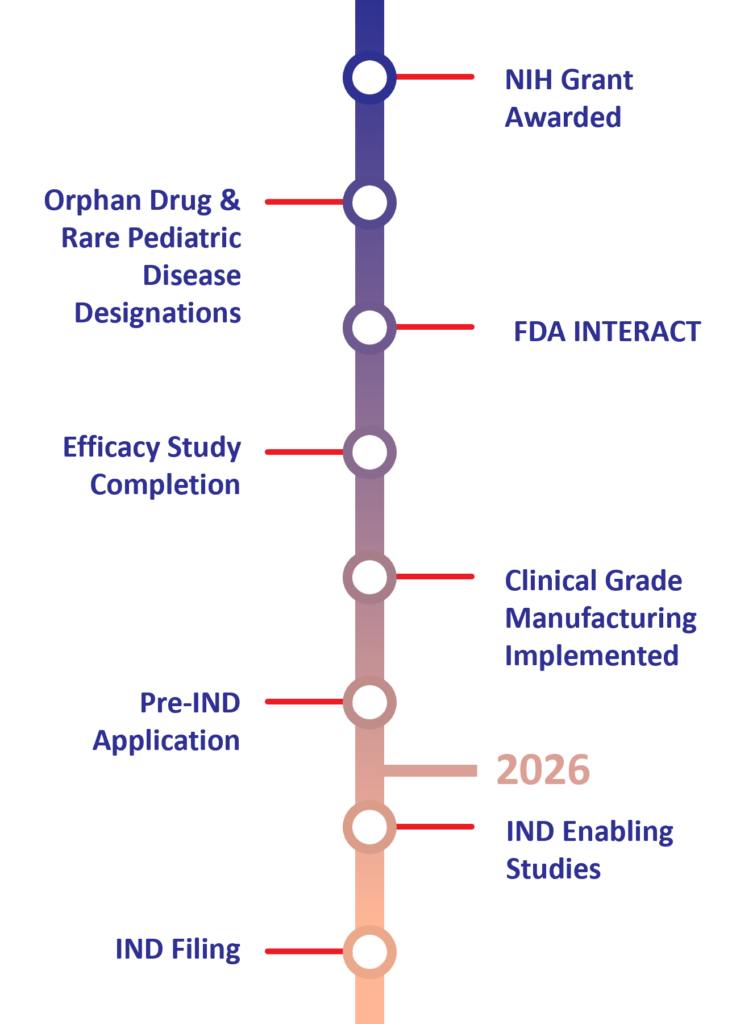
Human Placental Extract (HPE) Preclinical Milestones & Timeline for NEC

NEC and Pediatric Malabsorption
Pre-Clinical Efficacy
Reduction in NEC disease severity and number of affected regions in the gut with pre-treatment in a preclinical model of NEC.[1]
Mimicking Nature
High yield, consistent concentrations of proliferative, maturation, and anti-inflammatory factors necessary to drive gut development.[1][3]
Safety
Compilation of naturally existing proteins needed during normal development in utero, and no safety or tolerability incidences during preclinical evaluation to preclude the use of HPE among babies at risk for NEC.
Lower Hospital Costs
Cost analysis of Neonatal ICU cost of care show reduced time in the NICU, fewer surgeries, and lower long-term comprehensive care for affected neonates.[2]
Disclaimer: These statements have not been evaluated by the Food and Drug Administration.
References: (show)
- Data on file. Plakous Therapeutics.
- Mara KC, Clark RH, Carey WA. Necrotizing Enterocolitis in Very Low Birth Weight Neonates: A Natural History Study. Am J Perinatol. 2022 Sep 16. doi: 10.1055/a-1851-1692. PMID: 35554890.
- Frost BL, Modi BP, Jaksic T, Caplan MS. New Medical and Surgical Insights Into Neonatal Necrotizing Enterocolitis: A Review. JAMA Pediatr. 2017 Jan 1;171(1):83-88. doi: 10.1001/jamapediatrics.2016.2708. PMID: 27893069.
